No longer controversial: a new biological role for Z-DNA in cells
People gave up looking for left-handed Z-DNA-binding proteins long ago. This paper shows that many Z-DNA-binding proteins exist in a cell.
The cell is wired quite differently than imagined by the first generation of molecular biologists.”
CHARLESTOWN, MA, UNITED STATES, October 22, 2025 /EINPresswire.com/ -- Since its discovery, the role of left-handed DNA in cell biology has been a subject of controversy. Many believed that only the right-handed B-DNA helix, first described by Watson and Crick, provided the only cellular blueprint that Nature used. It was then surprising that the first synthetic DNA crystal was left-handed and had a zig-zag backbone rather than the smooth spiral of B-DNA. The left-handed DNA was called Z-DNA, but was quickly dismissed as biologically relevant. Now, nearly fifty years later, an essential role for Z-DNA in cellular biology has been revealed. In a paper published today in the Royal Society Open Biology, Dr. Herbert of InsideOutBio has provided evidence that many cellular proteins involved in controlling the readout of genes into RNA recognize Z-DNA, which allows them to scan the genome rapidly to reach their site of action. — Alan Herbert
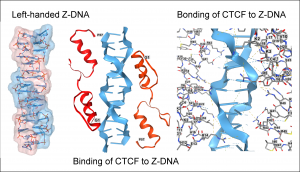
The proteins that bind Z-DNA described in the paper are called transcription factors. Those discussed belong to a family of over 800 transcription factors that contain zinc in their DNA-binding domain. These zinc-finger domains are quite short and quite ancient. They date back to the first life forms. They bind to right-handed B-DNA with high affinity and can recognize specific DNA sequences by engaging each base individually.
Previously, it was assumed that each zinc-finger domain only interacts with B-DNA. However, by use of computational modeling, Dr. Herbert revealed interaction modes with Z-DNA in which the domain binds to the Z-DNA structure rather than through recognition of each base. These findings show that the zinc fingers bind to both B-DNA and Z-DNA through the same domain. The nature of the interaction differs. The binding to Z-DNA is structure-specific rather than sequence-specific. The sequences that form Z-DNA inside cells are called flipons. They can transition between B-DNA and Z-DNA without a change in sequence or a scission of the DNA backbone.
Z-DNA is a higher-energy DNA conformation in which the base-pairs are flipped to create its distinctive structure. It is formed in active parts of the genome, with enzymes like RNA polymerase powering the transition. The presence of Z-DNA flags those regions that control the production of RNA from DNA. Since the formation of Z-DNA is transient, the binding of zinc-finger domains to this conformation has different characteristics compared to their interaction with B-DNA.
The work resolves a long-standing paradox. Zinc-finger proteins bind to B-DNA sequences very tightly, but the 3 to 9 base combination they recognize is quite frequent in the genome. This raises the question of how these proteins find the “right” sequence to engage in a genome that has 3 billion base pairs. To do so by testing each sequence individually would take too long for a cell to respond rapidly to a change in circumstance. The zinc finger proteins risk being trapped in the wrong place due to their strong interaction with B-DNA.
In contrast, the binding to Z-DNA is more transient. By searching for Z-DNA, these transcription factors have found a faster way to scan the genome for the “right” sequence. The Z-DNA localizes these proteins to active parts of the genome. These regions contain the B-DNA sequences that the proteins engage to control the readout of DNA into RNA. The recognition of Z-DNA solves the scanning problem. The proteins can move from one Z-DNA-forming region to another until they find the “right” DNA sequence to engage. They then create a stable complex with DNA that allows the assembly of the required cellular machinery at that site. This machinery may sometimes increase or suppress transcription. The outcome depends on whether activating or inhibitory domains are present in the zinc finger protein.
Besides transcription, zinc-finger proteins also impact how different chromosomal segments are arranged in the cell nucleus. This outcome is possible because many proteins incorporate multiple zinc-finger domains. This design allows the proteins to bridge widely separated chromosomal regions. The search for such connections is made more efficient by first scanning for Z-DNA to find a specific B-DNA binding site at each location. Since each zinc finger domain may prefer a different B-DNA sequence, the bridge formed between these chromosomal segments has well-defined anchor points. By creating different bridges that vary with the B-DNA sequence-specificity of the zinc finger domains involved, a protein can vary the arrangement of chromosomes in a cell. The bridging of different chromosomal segments in each cell type leads to the transcription of tissue-specific gene sets. Dr Herbert provides examples of such interactions in the paper.
It is well known in science that researchers often focus on a particular problem and become fixed in their understanding of how a cell works. The discovery that zinc finger proteins bind alternative DNA conformations could have been made many times over the last 50 years, but no one thought to look. Indeed, the expectation that none existed became for many a self-fulfilling prophecy. The assays used by others to find Z-DNA binding proteins were designed on the assumption that distinct protein domains bound to B-DNA and Z-DNA conformations. Hence, the search for Z-DNA-specific proteins included a B-DNA competitor to mask B-DNA binding proteins. Unfortunately, this approach also masked those domains that bind to both B- and Z-DNA conformations.
InsideOutBio is a start-up focused on developing a novel class of proprietary therapeutics to ‘light up tumors for the immune system. These statements about InsideOutBio comply with Safe-Harbor laws. They are forward-looking and involve known and unknown risks and uncertainties. They are not guarantees of future performance, and undue reliance should not be placed on them.
Alan Herbert
InsideOutBio, Inc
+1 617-800-7531
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.